Preservative Efficacy Testing: Executive Summary
Cost Range
€300-€700
Per product, depending on method and lab
Timeline
4-6 Weeks
From sample receipt to final report
EU Method
ISO 11930
Recommended for EU/UK market entry
Required For
CPSR
Cosmetic Product Safety Report
Key Takeaway: If you are launching a water-containing cosmetic product in the EU or UK market, you will need preservative efficacy testing as part of your Cosmetic Product Safety Report (CPSR). ISO 11930 is the standard method accepted by EU safety assessors. Testing typically costs €410-€700 and takes 4-6 weeks.
Quick Answers: Jump to Frequently Asked Questions for cost, timeline, and method selection guidance.
What is Preservative Efficacy Testing?
Preservative efficacy testing, commonly abbreviated as PET and also known as challenge testing or antimicrobial effectiveness testing, is a laboratory procedure that evaluates whether a cosmetic product's preservative system can adequately protect against microbial contamination. This testing is essential for any cosmetic product containing water, as water provides the environment necessary for bacteria, yeast, and mold to grow.
The fundamental principle behind preservative efficacy testing involves deliberately contaminating the product with known quantities of specific microorganisms, then monitoring whether the preservative system can reduce and control these populations over a defined period. For cosmetic products destined for the EU market, this testing forms a critical component of the safety documentation required under EU Regulation 1223/2009.
How Preservative Efficacy Testing Works
During a preservative efficacy test, laboratory technicians inoculate samples of your cosmetic product with standardized concentrations of challenge organisms. The test protocol then requires sampling at specific intervals, typically Day 0, Day 7, Day 14, and Day 28, to measure the surviving microbial population. The preservative system passes if it achieves the required log reductions specified by the test method.
The challenge organisms used in preservative efficacy testing represent the types of contamination most likely to occur during normal product use. These include gram-negative bacteria like Pseudomonas aeruginosa and Escherichia coli, gram-positive bacteria such as Staphylococcus aureus, the yeast Candida albicans, and the mold Aspergillus brasiliensis. A robust preservative system must demonstrate effectiveness against all these organism types.
Why Preservative Efficacy Testing Matters for EU Compliance
Under EU Regulation 1223/2009, every cosmetic product placed on the EU market must have a Cosmetic Product Safety Report (CPSR) prepared by a qualified safety assessor. The CPSR must include evidence that the product is microbiologically safe, and for water-containing products, this evidence comes from preservative efficacy testing. Without a valid PET report, your safety assessor cannot complete the CPSR, and you cannot legally sell your product in the EU.
The requirement extends beyond just having a preservative in your formula. You must demonstrate that your specific formulation, with its unique combination of ingredients, pH, and water activity, provides adequate antimicrobial protection. Factors such as other ingredients, packaging type, and intended use conditions can all affect preservative performance, which is why testing the finished product is essential.
Who Needs Preservative Efficacy Testing?
Preservative efficacy testing is required for cosmetic brand founders launching water-containing products such as creams, lotions, serums, and shampoos. Private label manufacturers creating custom formulations need testing for each unique formula. E-commerce sellers importing cosmetics into EU and UK markets must ensure their products have valid PET documentation. Contract manufacturers producing for multiple brands typically require separate testing for each client's formulation.
Real-World Example: The Cost of Skipping PET
A skincare brand launched a vitamin C serum without preservative efficacy testing, relying on the assumption that the low pH would prevent microbial growth. Within three months, customer complaints about product discoloration and unusual odor led to a product recall. Laboratory analysis revealed bacterial contamination. The brand faced costs exceeding €58,000 including product recalls, refunds, replacement testing, reformulation, and reputational damage. Proper preservative efficacy testing would have cost approximately €470 and identified the inadequate preservation before launch.
Video: Understanding Preservative Efficacy Testing
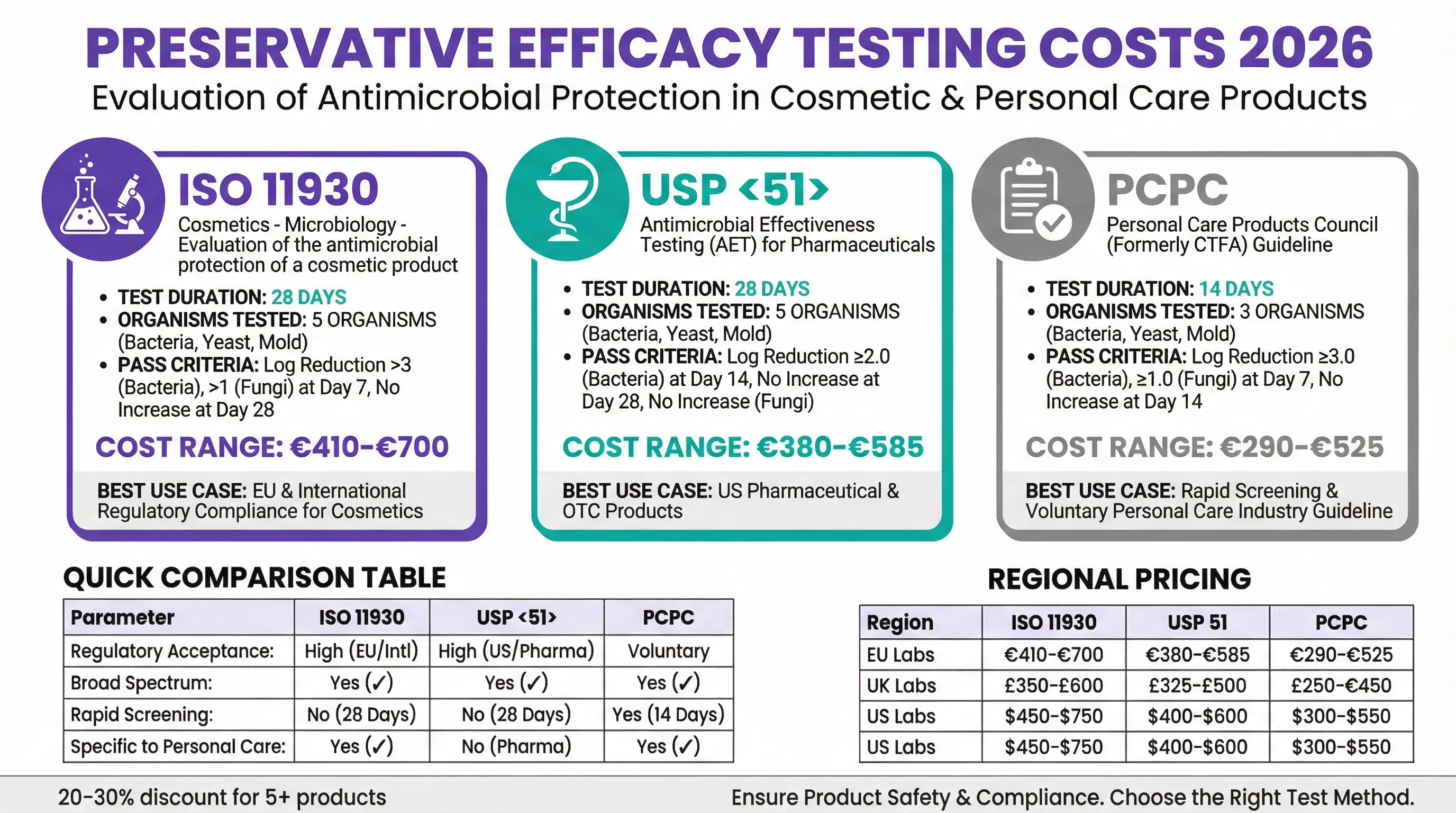
ISO 11930 vs USP 51 vs PCPC: Preservative Efficacy Test Method Comparison
Three primary test methods exist for evaluating preservative efficacy in cosmetic and personal care products: ISO 11930, USP 51, and the PCPC method. Each has distinct characteristics, acceptance criteria, and regulatory recognition. Understanding these differences is essential for selecting the appropriate test for your target market and product type.
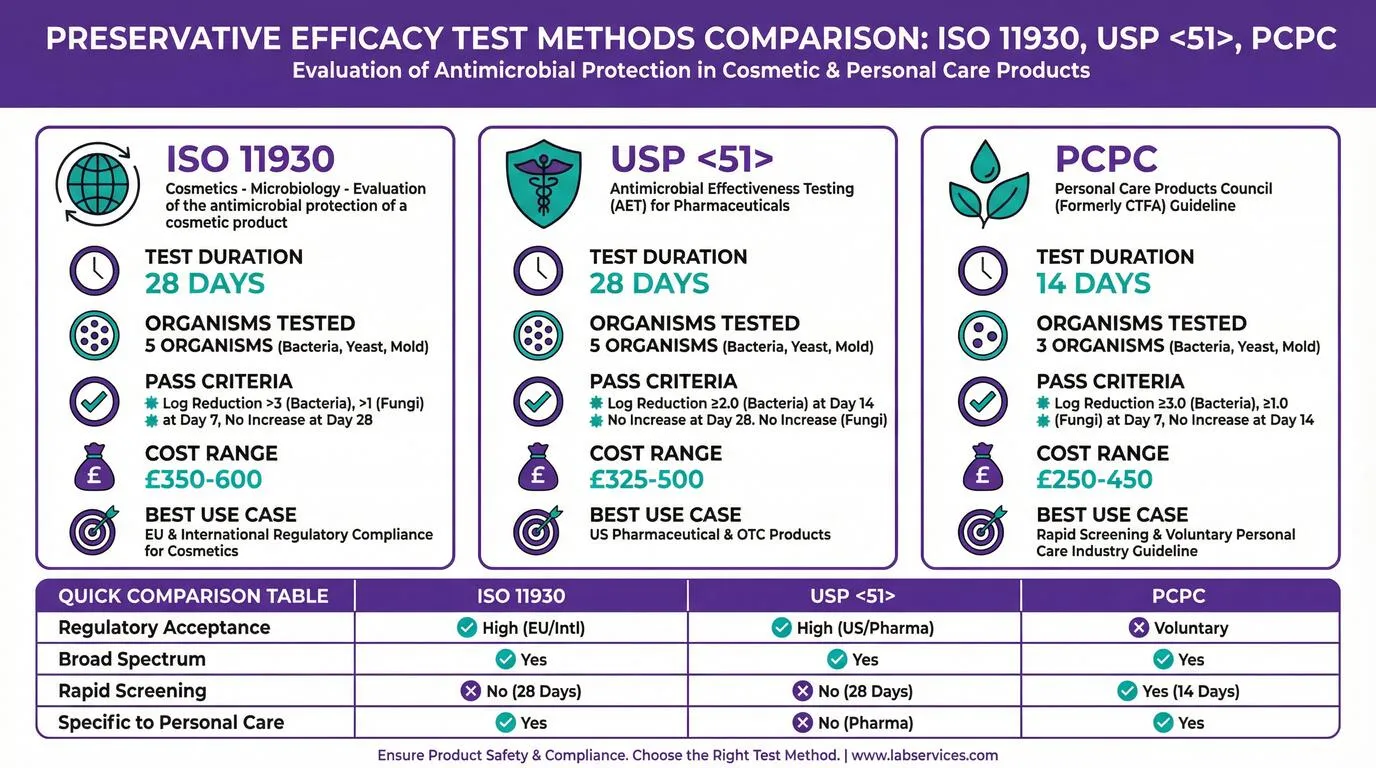
ISO 11930: The EU Standard for Cosmetics
ISO 11930 is the international standard specifically developed for evaluating the antimicrobial protection of cosmetic products. Published by the International Organization for Standardization, this method is the preferred choice for products entering the EU and UK markets. The test runs for 28 days and challenges products against five microorganisms: three bacteria (Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli), one yeast (Candida albicans), and one mold (Aspergillus brasiliensis).
The pass criteria for ISO 11930 require specific log reductions at defined time points. For bacteria, products must achieve a 3-log reduction by Day 7 with no increase through Day 28. For yeast and mold, a 1-log reduction by Day 7 with no increase through Day 28 is required. Products meeting these criteria receive a Category A pass, indicating robust preservation. A Category B pass indicates adequate preservation under normal use conditions.
USP 51: The Pharmaceutical Standard
USP 51, published in the United States Pharmacopeia, was originally developed for pharmaceutical products but is sometimes applied to cosmetics, particularly those sold in the US market. The test duration is also 28 days, testing against the same five organism types as ISO 11930. However, the acceptance criteria differ, with USP 51 requiring a 2-log reduction for bacteria at Day 14 and no increase at Day 28.
While USP 51 is widely recognized in the pharmaceutical industry, EU safety assessors generally prefer ISO 11930 for cosmetic products because it was specifically designed for this product category. If you plan to sell primarily in the US market or have products that bridge the cosmetic-pharmaceutical boundary, USP 51 may be appropriate.
PCPC Method: Industry Guideline
The Personal Care Products Council (PCPC) method, formerly known as the CTFA method, is a voluntary industry guideline rather than an official standard. It offers a shorter 14-day test duration and tests against only three organisms, making it faster and less expensive than ISO 11930 or USP 51. However, its voluntary status means it may not be accepted by all safety assessors or regulatory authorities.
The PCPC method is best suited for rapid screening during product development or for products sold in markets with less stringent regulatory requirements. For EU market entry, ISO 11930 remains the recommended choice.
Detailed Method Comparison Table
| Parameter | ISO 11930 | USP 51 | PCPC |
|---|---|---|---|
| Test Duration | 28 days | 28 days | 14 days |
| Organisms Tested | 5 (3 bacteria, 1 yeast, 1 mold) | 5 (3 bacteria, 1 yeast, 1 mold) | 3 (2 bacteria, 1 yeast/mold) |
| Sampling Points | Days 0, 7, 14, 28 | Days 0, 7, 14, 28 | Days 0, 7, 14 |
| Pass Criteria (Bacteria) | ≥3 log reduction by Day 7 | ≥2 log reduction by Day 14 | ≥3 log reduction by Day 7 |
| Typical Cost (UK) | €410-€700 | €380-€585 | €290-€525 |
| Regulatory Recognition | EU, UK, International | US, Pharmaceutical | Voluntary/Industry |
| Best Use Case | EU/UK cosmetics compliance | US market, OTC products | Rapid screening, development |
Decision Flowchart: Choosing Your Test Method
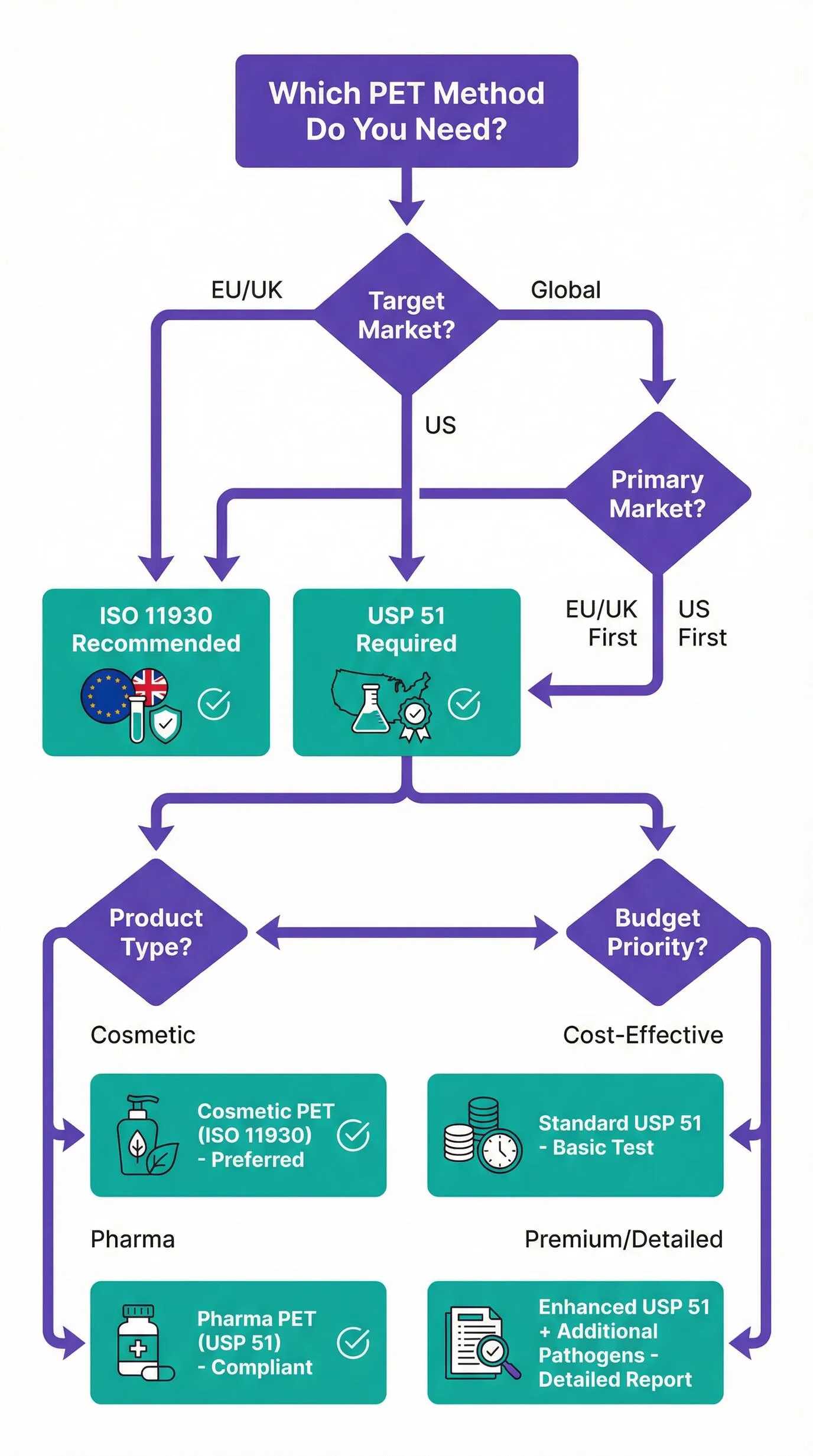
Recommendation for EU Market Entry
If your primary market is the EU or UK, choose ISO 11930 for your preservative efficacy testing. This method is specifically designed for cosmetic products, is referenced in EU regulatory guidance, and is universally accepted by CPSR assessors. While USP 51 may be accepted in some cases, ISO 11930 eliminates any potential questions about method suitability.
Preservative Efficacy Testing Timeline and Process
Understanding the preservative efficacy testing timeline is essential for planning your product launch. The complete process from sample submission to receiving your final report typically takes 4-6 weeks. This timeline is largely determined by the 28-day challenge test period required by ISO 11930 and USP 51 methods.
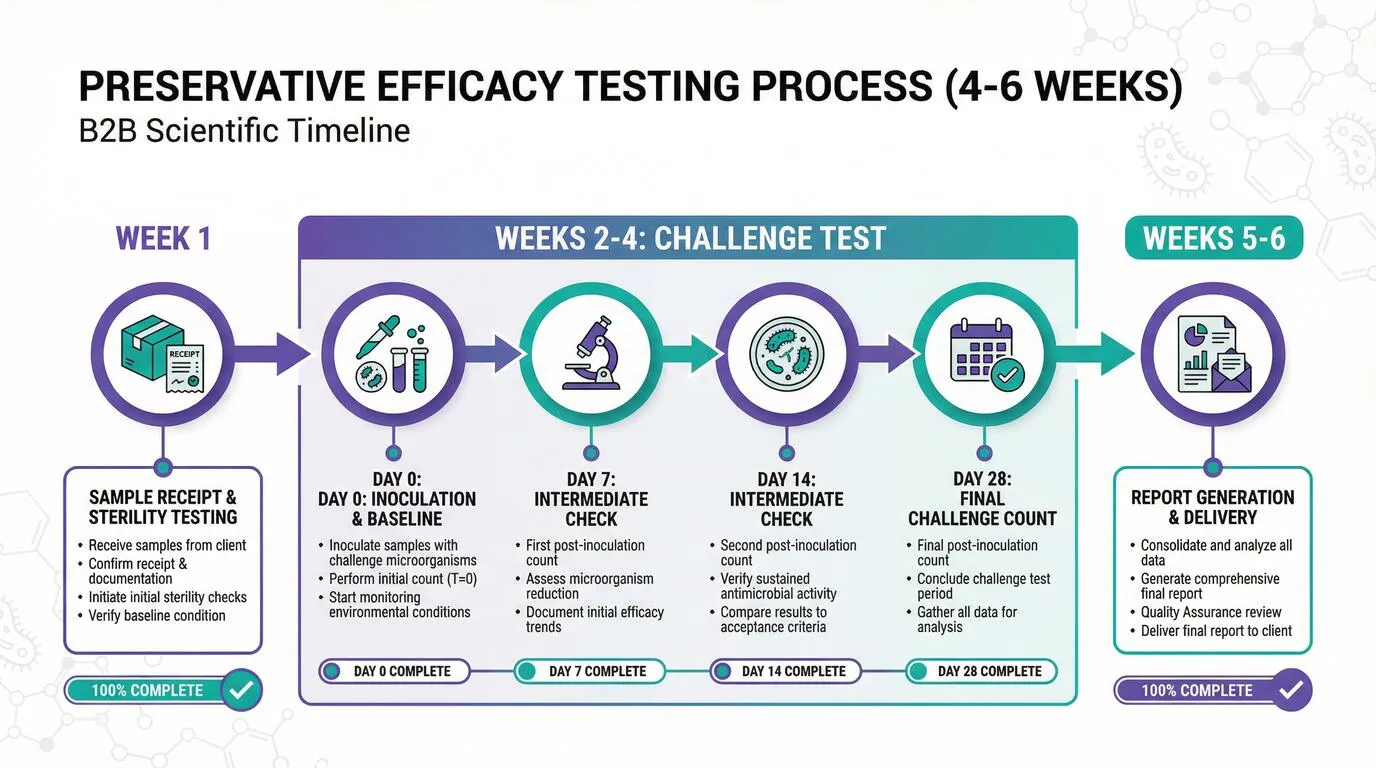
Week-by-Week Breakdown
Sample Receipt and Preparation
Upon receiving your samples, the laboratory conducts initial checks including sample integrity verification, documentation review, and baseline sterility testing. The sterility test confirms your product is not already contaminated before the challenge test begins. If contamination is detected, you will be notified immediately and testing cannot proceed until a clean sample is provided.
Challenge Test Period
The 28-day challenge test begins with inoculation of your product samples with standardized concentrations of the five test organisms. Sampling occurs at Day 0 (immediately after inoculation), Day 7, Day 14, and Day 28. At each sampling point, technicians enumerate the surviving microorganisms to track the preservative system's effectiveness over time.
Analysis and Report Generation
After the Day 28 sampling, the laboratory analyzes all results, calculates log reductions, and determines pass/fail status against the relevant criteria. A comprehensive report is prepared detailing the methodology, results for each organism, and the overall assessment. Most laboratories deliver reports within 5-7 working days after test completion.
Rush Service Options
While the 28-day challenge test period cannot be shortened without compromising scientific validity, some laboratories offer expedited processing for the administrative and analytical phases. Rush services may reduce the overall timeline by 3-5 days and typically incur an additional fee of 25-50% above standard pricing. The PCPC method, with its 14-day test duration, offers a faster alternative for situations where this method is acceptable.
Common Causes of Delays
Several factors can extend your preservative efficacy testing timeline beyond the standard 4-6 weeks. Insufficient sample quantity is a frequent issue; laboratories typically require 100-150g of product to complete all testing. Incomplete documentation, particularly missing INCI lists or pH values, can delay test initiation. Products arriving contaminated require reformulation and resubmission. Planning for these potential issues by providing complete documentation and adequate sample quantities helps ensure your testing stays on schedule.
Anhydrous Products and Preservative Efficacy Testing
A common question from cosmetic formulators concerns whether anhydrous (water-free) products require preservative efficacy testing. The short answer is that truly anhydrous products with low water activity typically do not need preservative testing, but determining whether your product qualifies requires understanding water activity and its measurement.
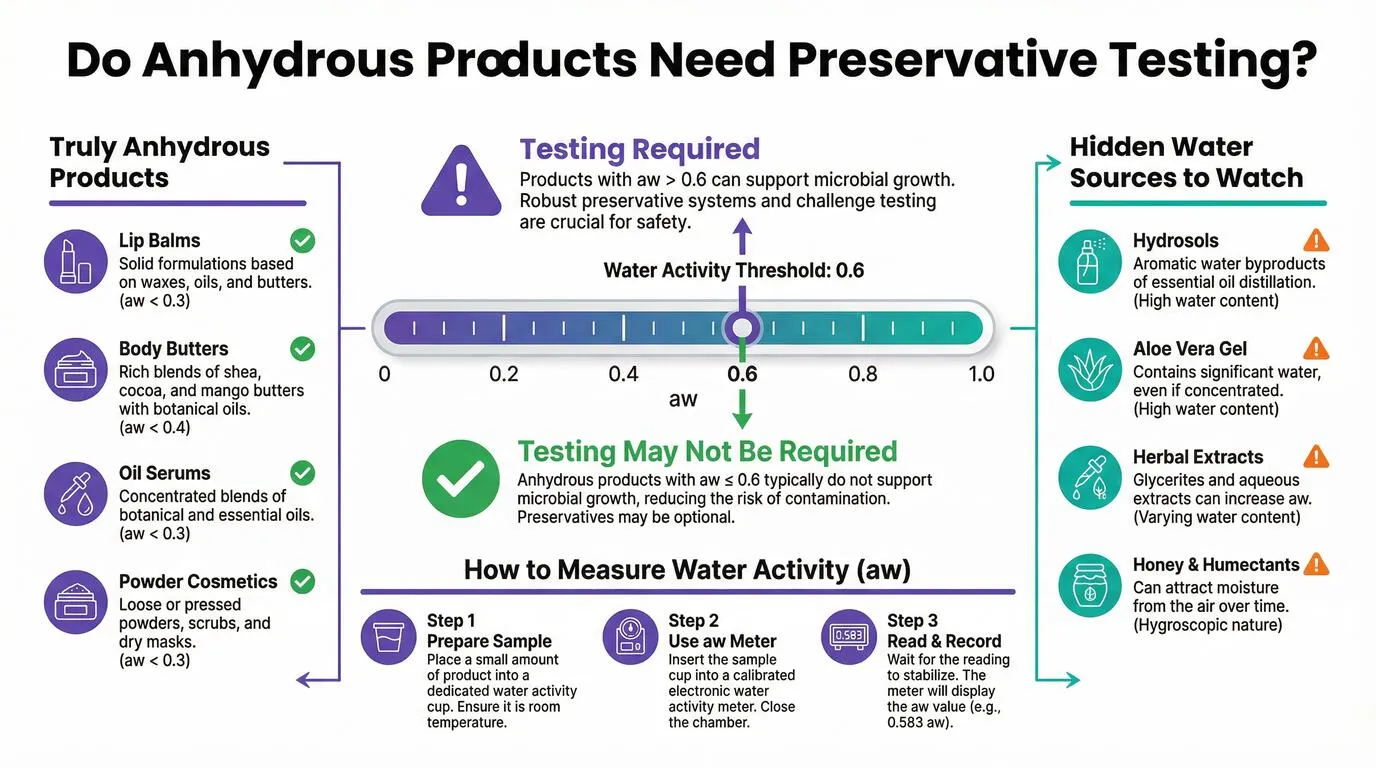
Understanding Water Activity
Water activity (aw) measures the availability of water in a product for microbial growth, expressed on a scale from 0 to 1. Pure water has an aw of 1.0, while completely dry products approach 0. Microorganisms require a minimum water activity to survive and reproduce. Most bacteria need aw above 0.9, yeasts above 0.85, and molds above 0.7. Products with water activity below 0.6 are generally considered unable to support microbial growth.
The critical threshold for cosmetic products is typically aw 0.6. Products below this level are unlikely to support microbial contamination and may be exempt from preservative efficacy testing. However, this exemption applies only to products that will remain below this threshold throughout their shelf life and use period.
When Oil-Based Products Still Need Testing
Not all products marketed as "oil-based" or "anhydrous" are truly water-free. Many formulations contain ingredients that introduce water or have high water activity themselves. Hydrosols and floral waters are essentially water with dissolved plant compounds. Aloe vera gel, even when concentrated, contains significant water. Glycerin and other humectants can attract moisture from the environment. Aqueous herbal extracts, even at low concentrations, introduce water into the formula.
If your formula contains any of these ingredients, or if you are uncertain about the water activity of your finished product, testing is recommended. A water activity measurement costs significantly less than preservative efficacy testing and can definitively determine whether your product requires the full challenge test.
pH-Based Considerations
Extreme pH values can also inhibit microbial growth. Products with pH below 3.5 or above 10 may have inherent antimicrobial properties that reduce or eliminate the need for added preservatives. However, pH alone is not sufficient justification to skip preservative efficacy testing. The combination of pH, water activity, and other formula factors must be evaluated by a qualified safety assessor to determine testing requirements.
How to Measure Water Activity
Water activity measurement requires specialized equipment called a water activity meter. These devices equilibrate a sample in a sealed chamber and measure the relative humidity, which corresponds to water activity. Professional laboratories offer water activity testing as a standalone service, typically costing €35-€60 per sample. For formulators developing multiple products, investing in a benchtop water activity meter may be cost-effective.
Products Typically Exempt from Preservative Efficacy Testing
- Lip balms formulated entirely from waxes, oils, and butters
- Body butters without aqueous ingredients
- Anhydrous oil serums and facial oils
- Solid perfumes and balm-based fragrances
- Powder products including loose and pressed powders
- Bar soaps with very low moisture content
Sample Preparation and Shipping for Preservative Efficacy Testing
Proper sample preparation is essential for accurate preservative efficacy testing results. Laboratories have specific requirements for sample quantity, packaging, labeling, and documentation. Following these guidelines ensures your samples arrive in optimal condition and testing can begin without delays.
Sample Quantity Requirements
Most laboratories require a minimum of 100g of product for preservative efficacy testing. This quantity allows for testing against all five organisms with sufficient replicates and reserve samples. For products in small containers, you may need to provide multiple units totaling the required amount. Some laboratories request up to 150g to ensure adequate material for any necessary repeat testing.
Packaging Guidelines
Samples should be provided in their final market packaging whenever possible, as packaging can affect preservative stability and product contamination risk. If final packaging is not available, use clean, airtight containers appropriate for your product type. Glass or high-quality plastic containers with secure closures are preferred. Avoid containers that may react with your product or allow contamination during transit.
Labeling Requirements
Each sample container must be clearly labeled with the product name or code, batch number, manufacturing date, and your company name or reference number. Labels should be waterproof and securely attached. If submitting multiple products, ensure each is distinctly identified to prevent confusion during testing.
Required Documentation
Complete documentation accelerates test initiation and ensures accurate reporting. Laboratories typically require the full INCI ingredient list with percentages, product pH value, preservative system details including type and concentration, intended product use and application method, and any relevant stability data. Providing comprehensive documentation upfront prevents delays from follow-up requests.
Shipping Best Practices
Ship samples using a tracked courier service to ensure delivery confirmation and enable tracking if issues arise. Package samples securely to prevent breakage or leakage during transit. Include cushioning material around containers and use leak-proof outer packaging. For temperature-sensitive products, consider insulated packaging or cold packs, particularly during summer months.
Sample Submission Checklist
- Minimum 100g of product (check laboratory requirements)
- Product in final packaging or appropriate containers
- Clear, waterproof labels on all containers
- Complete INCI list with percentages
- Product pH value
- Preservative type and concentration
- Completed laboratory submission form
- Purchase order or payment confirmation
- Secure, leak-proof outer packaging
- Tracked shipping with delivery confirmation
What is Included in a Preservative Efficacy Test Report?
Upon completion of preservative efficacy testing, you will receive a comprehensive report documenting the test methodology, results, and conclusions. Understanding the components of this report helps you interpret the findings and communicate effectively with your safety assessor.
Standard Report Components
A complete preservative efficacy test report includes several key sections. The header information identifies the testing laboratory, their accreditation status, and report reference numbers. Sample identification details your product name, batch number, and receipt date. The methodology section specifies the test standard used (ISO 11930, USP 51, or PCPC), including any deviations from the standard protocol.
The results section presents microbial counts at each sampling point for all test organisms. This data is typically shown in tabular format with colony-forming units (CFU) per gram or milliliter. Log reduction calculations demonstrate the preservative system's effectiveness over time. The conclusion states whether the product passed or failed the acceptance criteria, with Category A or B designation for ISO 11930 testing.
How Safety Assessors Use the Report
Your CPSR assessor reviews the preservative efficacy test report as part of the overall safety evaluation. They verify that the testing was conducted by an accredited laboratory using an appropriate method. The assessor evaluates whether the results demonstrate adequate preservation for your specific product type and intended use conditions. They may also consider factors such as packaging type, target consumer population, and product application method when interpreting results.
A passing result allows the assessor to conclude that your product's preservative system provides adequate protection against microbial contamination. This conclusion, combined with other safety data, supports the overall assessment that your product is safe for its intended use.
Pass/Fail Criteria Explained
For ISO 11930 testing, products are classified into categories based on their performance. Category A indicates the product meets the most stringent criteria, demonstrating robust preservation suitable for all cosmetic product types. Category B indicates adequate preservation for products with lower contamination risk, such as those with pump dispensers or single-use packaging. Products failing to meet Category B criteria do not pass and require reformulation.
What to Do with Your Report
Store your preservative efficacy test report securely as part of your Product Information File (PIF). The report must be available for regulatory inspection for the entire time your product is on the market plus ten years after the last batch is sold. Provide a copy to your safety assessor for inclusion in the CPSR. If you reformulate your product or change the preservative system, new testing will be required.
Video: How PET Fits Into Your CPSR
Preservative Efficacy Testing Pricing Guide 2026
Understanding preservative efficacy testing costs helps you budget accurately for product development and launch. Prices vary based on the test method, laboratory location, accreditation level, and additional services. This guide provides current pricing ranges to help you plan your testing investment.
Cost Breakdown by Method
ISO 11930 preservative efficacy testing typically costs between €410 and €700 per product at European laboratories. This range reflects differences in laboratory overhead, accreditation scope, and service levels. UK laboratories may charge £350-£600 for equivalent testing, while US laboratories offering ISO 11930 testing typically charge $450-$750.
USP 51 testing ranges from €380 to €585 at European laboratories, with similar pricing at UK facilities. US laboratories, where USP 51 is more commonly requested, typically charge $400-$600. The PCPC method, being shorter and testing fewer organisms, costs €290-€525 at most laboratories.
Regional Price Comparison
| Region | ISO 11930 | USP 51 | PCPC |
|---|---|---|---|
| EU Labs | €410-€700 | €380-€585 | €290-€525 |
| UK Labs | £350-£600 | £325-£500 | £250-£450 |
| US Labs | $450-$750 | $400-$600 | $300-$550 |
Factors Affecting Price
Several factors influence the final cost of preservative efficacy testing. Laboratory accreditation level affects pricing, with ISO 17025 accredited laboratories typically charging premium rates that reflect their quality management systems and regulatory recognition. Geographic location impacts costs through differences in labor rates and operational expenses. Rush services add 25-50% to standard pricing. Complex products requiring modified protocols may incur additional charges.
Batch Discounts
Most laboratories offer significant discounts for testing multiple products simultaneously. Batch discounts typically range from 20-30% when submitting five or more products together. For brands launching product ranges, coordinating testing can substantially reduce per-product costs. Some laboratories also offer annual testing agreements with preferential pricing for regular clients.
Cost Optimization Strategies
- Submit multiple products together to qualify for batch discounts
- Plan testing early to avoid rush fees
- Provide complete documentation to prevent delays and additional charges
- Consider PCPC method for development screening before final ISO 11930 testing
- Negotiate annual agreements if launching multiple products per year
Frequently Asked Questions About Preservative Efficacy Testing
Preservative efficacy testing (PET), also called challenge testing, evaluates whether a cosmetic product's preservative system can effectively prevent microbial growth. The test involves deliberately inoculating the product with specific microorganisms and monitoring their reduction over 28 days. PET is required for water-containing cosmetics sold in the EU as part of the Cosmetic Product Safety Report (CPSR) under Regulation 1223/2009.
Preservative efficacy testing typically costs between €300 and €700 per product, depending on the test method and laboratory. ISO 11930 testing ranges from €410-€700, USP 51 from €380-€585, and PCPC from €290-€525. Batch discounts of 20-30% are commonly available when testing 5 or more products simultaneously. Factors affecting price include laboratory accreditation level, geographic location, and any rush service requirements.
Standard preservative efficacy testing takes 4-6 weeks from sample receipt to final report delivery. The challenge test itself runs for 28 days with sampling at Days 0, 7, 14, and 28. Additional time is needed for initial sterility testing (Week 1) and report generation (Weeks 5-6). Rush services may reduce administrative processing time but cannot shorten the 28-day test period required by ISO 11930 and USP 51 methods.
For the EU market, ISO 11930 is the recommended and most widely accepted test method for cosmetic products. It is specifically designed for cosmetics and is referenced in EU regulatory guidance. Safety assessors preparing CPSRs universally accept ISO 11930 results. USP 51 is primarily used for pharmaceutical products in the US market, while PCPC is a voluntary industry guideline suitable for rapid screening during development.
Truly anhydrous products with water activity (aw) below 0.6 typically do not require preservative efficacy testing, as they cannot support microbial growth. However, products containing hidden water sources such as hydrosols, aloe vera gel, or aqueous herbal extracts may still need testing. A water activity measurement (approximately €35-€60) can confirm whether your product is exempt from preservative efficacy testing.
ISO 11930 tests against five microorganisms representing common contaminants: Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli (bacteria), Candida albicans (yeast), and Aspergillus brasiliensis (mold). These organisms are selected because they represent contamination types that could be introduced during product manufacturing or consumer use. The product must demonstrate adequate log reductions against all organisms to pass.
Generally, each unique formulation requires its own preservative efficacy test. However, if you have products with identical preservative systems and only minor variations (such as different fragrance or colorant at low concentrations), your safety assessor may accept bracketing or read-across arguments. This approach requires scientific justification that the variations do not affect preservative efficacy. Discuss this with your assessor before assuming a single test will cover multiple products.
If your product fails PET, you will need to reformulate the preservative system and retest. Common solutions include increasing preservative concentration within regulatory limits, adding a secondary or booster preservative, adjusting pH to optimize preservative activity, or reducing water activity through formula modification. Your laboratory or formulation chemist can advise on the most appropriate approach based on which organisms showed inadequate reduction.
Yes, your manufacturer can handle CPNP registration if they are designated as your Responsible Person (RP). However, many brands prefer to act as their own RP or hire a third-party RP service to maintain control over their product notifications. Regardless of who performs the registration, the PET report and CPSR must be completed first.
CPNP registration does not expire, but it must be updated if there are significant changes to the product formulation, labeling, or packaging. Additionally, if the Responsible Person changes, the notification must be transferred or updated. Regular reviews ensure ongoing compliance with evolving regulations.
No. The same ISO 11930 test report is accepted for both UK and EU Cosmetic Product Safety Reports (CPSR). However, you will need separate CPSRs and Responsible Persons for UK (SCPN) and EU (CPNP) registrations. The preservative efficacy test is the same for both markets.
Preservative efficacy testing should be done once your formula is finalized but before large-scale production. Ideally, test during the pilot batch phase. If your product fails PET, you'll need to reformulate and retest, which can delay launch by 4-6 weeks. Some brands do preliminary screening with the faster PCPC method (2 weeks) before final ISO 11930 testing.
Official Resources and Regulatory Bodies
Accurate information about preservative efficacy testing requirements comes from official regulatory sources and standards organizations. The following resources provide authoritative guidance on cosmetic safety requirements, test methods, and laboratory accreditation.
EU Regulations and Guidance
EU Regulation 1223/2009 on Cosmetic Products
The primary legislation governing cosmetic products in the European Union. Establishes requirements for safety assessment, labeling, and market notification.
EUR-Lex Summary: Safer Cosmetics in the EU
Plain-language summary of EU cosmetic regulations and their objectives for consumer safety.
European Commission: Cosmetics Sector
Official EU Commission page with guidance documents, updates on regulatory changes, and links to the CPNP notification portal.
UK Post-Brexit Guidance
UK Legislation: Retained EU Regulation 1223/2009
The EU Cosmetics Regulation as retained in UK law, with amendments reflecting the UK's departure from the EU.
UK Government: Cosmetic Products Regulation Guidance
Official UK government guidance on cosmetic product requirements, including notification to the UK responsible person.
Standards and Test Methods
ISO 11930:2019 - Cosmetics Microbiology
The international standard for evaluation of the antimicrobial protection of a cosmetic product. Available for purchase from ISO or national standards bodies.
United States Pharmacopeia (USP)
Publisher of USP 51 Antimicrobial Effectiveness Testing. Standards available through USP subscription services.
Personal Care Products Council (PCPC)
Industry association that publishes the PCPC (formerly CTFA) preservative efficacy test guideline.
Laboratory Accreditation Bodies
UKAS - United Kingdom Accreditation Service
The UK national accreditation body. Search their directory for ISO 17025 accredited laboratories offering cosmetic microbiology testing.
DAkkS - German Accreditation Body
Germany's national accreditation body for laboratories and certification bodies.
COFRAC - French Accreditation Committee
France's national accreditation body, recognized for laboratory accreditation across the EU.
Video: CPNP Registration Tutorial
Get Your Free Preservative Efficacy Testing Quote
Connect with ISO 17025-accredited laboratories and regulatory experts for your preservative efficacy testing needs. Complete the form below and receive a personalized quote within 24 hours.
Why Choose Our Partners
- ISO 17025-accredited lab partners
- 24-hour response time
- No obligation quote
- Expert regulatory guidance
- Batch discount available
Quick Reference
Typical Cost: €300-€700 per product
Timeline: 4-6 weeks
EU Method: ISO 11930
Sample Required: 100g minimum
